The mechanics of embryonic epithelia
PhD / Long term visitor's project
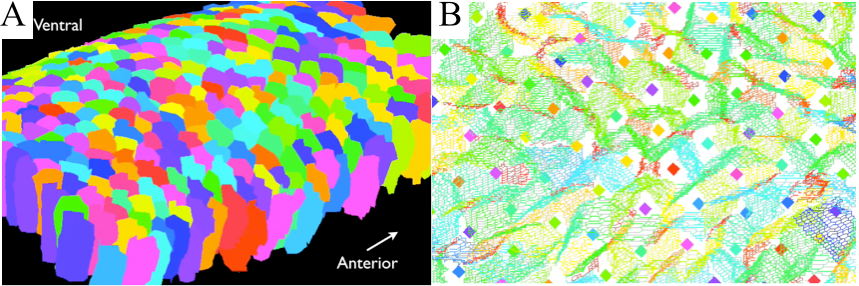
During the development of living organisms, large-scale morphogenetic movements of epithelial tissues occur, as observed during the contraction of Drosophila amnioserosa tissue or the convergence and extension of its germband tissue. It is now established that the main driving forces of such movements at the tissue scale originate from the molecular dynamics of the actin-myosin complex, where cross-linked polymer networks of actin are dragged by the action of the myosin motor-molecule.
Such behaviour of the actomyosin cytoskeleton was recently revealed to be mechano-responsive, i.e. influenced by external mechanical factors such as the epithelial tissue tension. In return, the response of the actomyosin network influences the mechanical properties of the cells and the tissue.
This project aims at developing the first explanatory model connecting the mechanistic understanding of sub-cellular actomyosin behaviour to the emergent mechanical properties of cells and tissues, and the way these evolve and self-regulate during morphogenetic movements.
The project is based on a two scale approach, combining state-of-the-art analyses of embryonic epithelial images with unique insights into the intracellular actomyosin molecular network.

CONTACTS
- PI: Guy Blanchard (visitor)
- Co-PI: Jocelyn Etienne
- PhD: Nilankur Dutta

PARTNERS
- Department of Physiology, Development and Neurosciences, University of Cambridge
- LIPhy

FUNDING
Tec21
