Mechanical removal of bacteria from surfaces: role of the extracellular matrix in resistance to decontamination
Half PhD grant
Dissemination of pathogenic microorganisms from the surface of contaminated equipment represents a major public health issue and can cause hospital-acquired infections or harmful food spoilage. Surface contamination by bacteria involves secreted extracellular polymeric substances (EPS) that mediate adhesion and into which they embed to form biofilms. In this state, they exhibit increased resistance to chemical, pharmaceutical or mechanical assault and strategies aiming at efficient removal of surface-attached bacteria often rely on the use of environmentally hazardous chemicals (washing at extreme pH or with toxic organic compounds). In this context, there is a strong drive to devise milder and greener strategies for surface decontamination, such as processes based on the mechanical action of a moving air/liquid interface and subsequent surface drying. Capillary forces generated on bacteria by the passage of an air-liquid meniscus are large enough to detach a fraction of cells, and complete drying is an efficient means of killing bacteria on surfaces. However, both detachment and drying may be hindered in the presence of microcolonies and EPS: a rational understanding of why/how a fraction of the adhered bacteria may resist a moving meniscus and subsequent drying have only been partly explored, and the behavior and fate of “mechanically resistant” bacteria have been left mostly unexplored.
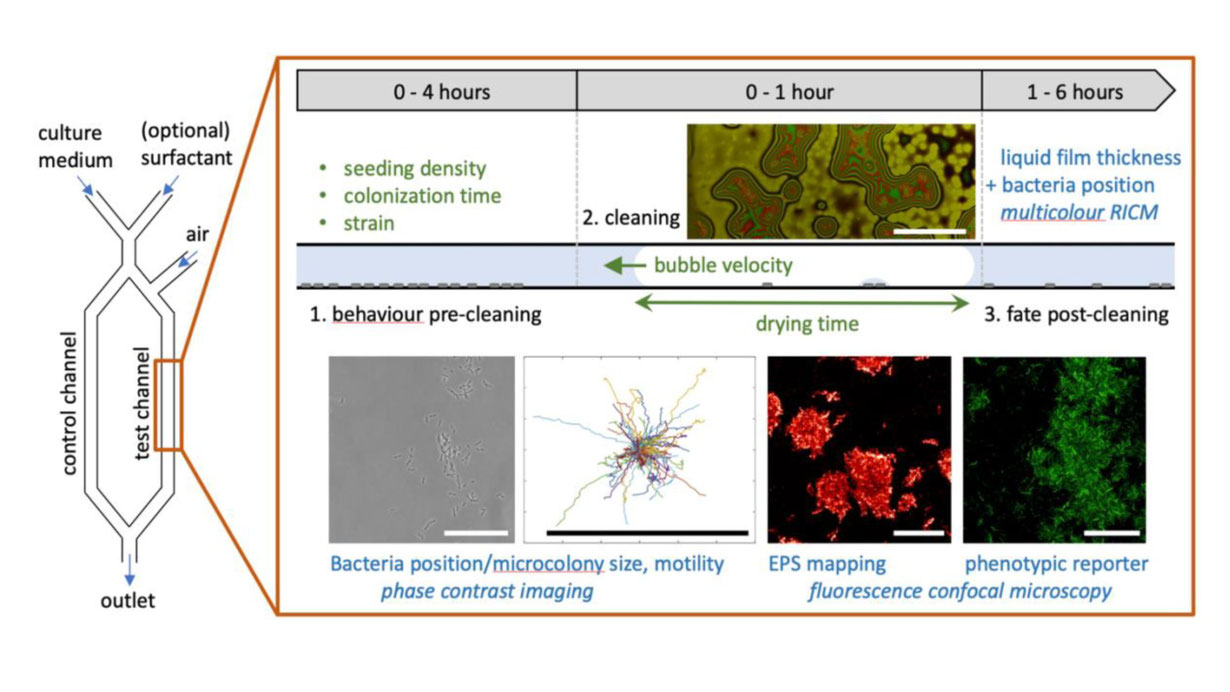
This project aims to investigate the physico-chemical aspects of bacterial resistance to mechanical decontamination, and how imperfect cleaning procedure may induce the emergence of mechanically-resistant bacteria through selective survival on the surface. Building on our experimental know-how in microbiology, microfluidics and microscopy, we will combine experimental assays with modelling of thin film hydrodynamics, dewetting, and drying of EPS gels, to (1) decipher quantitatively the physical role of EPS and bacteria clustering in bacterial survival to mechanical decontamination. Furthermore, combining decontamination assays with microbiology tools, we will (2) investigate whether populations of bacteria with particular phenotypes are selected by mechanical decontamination, and whether their phenotype is further modified post-decontamination to build up mechanical resistance. Integrating physical approaches with microbiology tools will improve our understanding of how to best harness mechanical forces for decontamination processes, but also of the limitations of this approach due to bacterial adaptation, and will pave the way to efficiently integrate mechanical approaches within chemical cleaning pipelines to minimize the use of harmful chemicals and improve the efficiency of milder biosurfactants.
FUNDING
Tec 21
Ecole Normale Supérieure de Lyon



