CELLUSWIMM- swimming vesicles as pilotable drug conveyors
Post-doc project
Most drug targeting strategies are based on concepts such as molecular recognition that could be considered as "passive" strategies. This project proposes to explore the possibilities of exploiting a physical phenomena called “buckling” to control the delivery of a drug in an active way.
When subjected to pressure cycles, air-filled spherical shells can “swim” in a liquid medium due to mechanical instability phenomena inducing the asymmetrical deformation of their membrane. A theoretical model was developed at the LIPhy to predict the swimming behaviour under different conditions, in particular the frequency and the amplitude of the pressure wave, the thickness of the shell membrane and its mechanical properties. This model shows that a micron-sized shell submitted to ultrasonic waves such as those emitted by echography devices, could swim at a speed that would enable the shell to travel upstream in blood circulation or in the lymphatic system.
This project consists in designing such shells on a micrometric scale by adsorbing cellulose and hemicellulose nanocrystals on the surface of micron-sized air bubbles produced in a microfluidic device, and to assemble them into an object whose displacement would be entirely controllable by ultrasonic waves. The existence of a biocompatible conveyor capable of moving through the body via ultrasound facilities would offer a unique solution for the vectorisation and dissemination of therapeutic molecules.
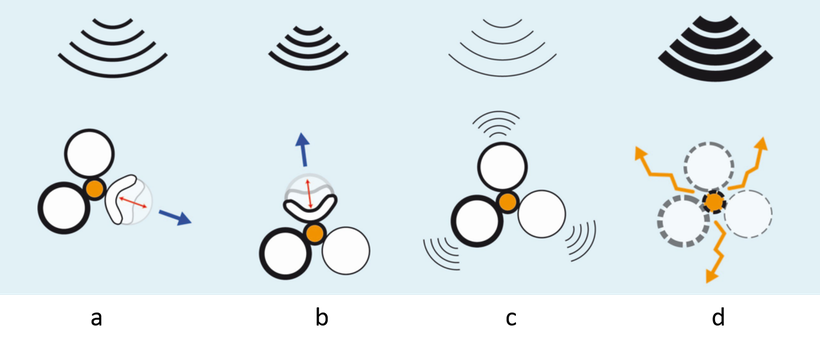
Figure caption: Because a single shell will undergo buckling in an unpredictable direction depending on its orientation and on the presence of mechanical weaknesses in its membrane, this project requires a strong know-how in the manipulation of micro-objects, in particular in the design of membranes with controlled thickness and composition. The idea is to produce a conveyor made of vesicles, each one reacting differently to an ultrasonic wave. The modulation of the frequency and wave length of the signal will selectively activate the buckling of each of the vesicles depending on their membrane thickness and/or material properties (a and b in the above figure), which will enable the operator to manage the orientation and pilot the movement of the conveyor. Note that the ultrasound wave produced by the echography device will also enable the operator to visualise the conveyor (c) and destroy it to release the drug (d)

CONTACTS
- PI: Catherine Quilliet
- Co-PI: Laurent Heux
- Post-doc: Candice Rey

PARTNERS
- LIPhy
- CERMAV

FUNDING
- Tec21
- Polynat
